Quantitative Chemistry
(GCSE Chemistry)
Firstly, it's important to know the law of conservation of mass. This is the law that states that mass cannot be created or destroyed. In reactions, no atoms can be created or destroyed, but combined to form new elements.
There are two main types of mass we need to know about:
Firstly, it's important to know the law of conservation of mass. This is the law that states that mass cannot be created or destroyed. In reactions, no atoms can be created or destroyed, but combined to form new elements.
 |
| (Louis Reed) |
There are two main types of mass we need to know about:
- Relative atomic mass- this is found by calculating the average mass from all of an elements isotopes.
- Relative formula mass- this is found by adding up the relative atomic masses of each element within the compound
To be able to see quantities of chemicals being used up for reactions, chemists came up with a larger unit to describe the number of particles a substance has. We call this unit a mole. We use this because it would be ridiculous to calculate equations using the number of particles. One mole is equal to Avogadro's number. 1 Mol= 602,200,000,000,000,000,000,000 particles or 6.022 × 1023
Our first equation here is;
Our second equation is:
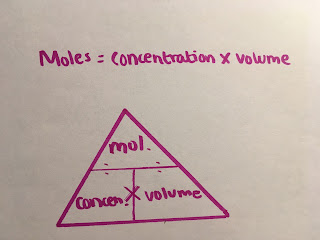
We can also calculate the concentration of a solute dissolved in a solvent by looking at the volume of the solution and the moles used in the reaction formula. Concentration is measured in mol/dm3 .Volume is measured in dm3 If you didn't know already, a decimetre is a thousand cubic centimetres (we use this when because we don't like handling large numbers!)
Our third equation is:
Atom economy of a reaction is the amount of the starting materials that end up as the desired product. It's in a company's best interest to use pathways of reactions that provide the highest atom economy so that there is the largest amount of desired product to use.
We can calculate the atom economy using the equation;
Percentage yield is the maximum possible mass of a desired product that can be made in a reaction. Again, the higher the percentage yield of a desired product, the better. We can calculate the percentage yield from;
- The balanced equation
- the mass and rel. formula mass of the limiting reactant
- the relative formula mass of the product
Although mass cannot be created or destroyed, it can be lost in a number of ways:
- By products/side products- the reactants can react with other, external substances to produce other, waste products.
- Slow reactions can cause the reactants to not react fully
- Reversible reactions can mean that the products can begin to convert back into the original reactants
Percentage yield can be calculated using the below equation:
If there is 100% percentage yield, no product is lost. If there is 0% percentage yield, no product is made. As mentioned, a company should try to use a reaction with the highest possible percentage yield in order to be cost-effective and waste as little as possible.
Our final 'yield' idea is called the maximum theoretical yield. The maximum theoretical yield is the highest possible mass of a product we can produce. This would be in an (unrealistic) ideal situation where no mass it lost as waste, which is what we often assume when calculating using chemical formulas.
Titration is an experiment carried out in order to determine the unknown concentration of an acid or base by using the known concentration of its opposite reactant. To put it simply, we use the reaction equation and the volumes used to neutralise to calculate the concentration of the unknown solution. This many sound complicated now, but once you understand it, it can be easy to understand and recall. 30cm3
In titrations, we place the substance with the known concentration into a burette using a volumetric pipette (for precision). We then place a set volume (for example ) into a beaker directly below the burette. Then we place an indicator like phenolphthalein which is clear in acid and pink in alkaline solutions. We could also use methyl orange (I find this much easier to spell and remember) which is red in acid, light orange in alkali and red-ish orange in neutral solutions. We then begin to slowly allow small amounts of the burette reactant (often the alkali) until the indicator shows the solution to have changed to alkaline/basic.
We can then use the calculate the moles used of the known solution by multiplying the concentration by the volume used to titrate the solution. Once we have calculated the moles used of the known solution, we can use the balanced equation to calculate the moles of the chemical with the unknown concentration. Once we know the moles, we can divide the moles by the volume to calculate the concentration.
In titrations, we place the substance with the known concentration into a burette using a volumetric pipette (for precision). We then place a set volume (for example ) into a beaker directly below the burette. Then we place an indicator like phenolphthalein which is clear in acid and pink in alkaline solutions. We could also use methyl orange (I find this much easier to spell and remember) which is red in acid, light orange in alkali and red-ish orange in neutral solutions. We then begin to slowly allow small amounts of the burette reactant (often the alkali) until the indicator shows the solution to have changed to alkaline/basic.
We can then use the calculate the moles used of the known solution by multiplying the concentration by the volume used to titrate the solution. Once we have calculated the moles used of the known solution, we can use the balanced equation to calculate the moles of the chemical with the unknown concentration. Once we know the moles, we can divide the moles by the volume to calculate the concentration.






Comments
Post a Comment